Surface Electromyographic Biofeedback of Pelvic Floor Musculature
Biofeedback is a self regulation training technique derived from well established principles of human learning. Biofeedback is a technique, not a stand alone treatment, which is one component of a behavioral training program to facilitate acquisition of pelvic floor muscle control and other continence skills.
Introduction
Biofeedback is a self regulation training technique derived from well established principles of human learning. Biofeedback is a technique, not a stand alone treatment, which is one component of a behavioral training program to facilitate acquisition of pelvic floor muscle control and other continence skills. The purpose of this chapter is to familiarize the reader with various methods of biofeedback training for urinary and fecal incontinence or retention and their practical implementation in the office practice setting.
With the use of biofeedback, physiological change can be achieved by means of operant conditioning, a type of learning which occurs as a result of feedback, or the experience and awareness of the consequences of one’s behavior. The first step in using biofeedback as a therapeutic tool is to understand the anatomy and physiology underlying the symptomatic dysfunction. This allows the selection and measurement, and ultimately voluntary control, of a physiological response. In this chapter we are dealing with urinary and defecatory dysfunctions, primarily incontinence, or involuntary loss, but also disorders of retention or inability to void. Bowel and bladder storage and voiding are both complex physiological events with many controlling factors as described in the following sections. While it is possible to measure the pressure changes that result from the activity of the bladder and bowel using intravesical or rectal manometry respectively, these procedures are not well adapted to office practice, particularly by non medical biofeedback practitioners. Therefore we will restrict ourselves in this chapter to dealing with external urethral and anal sphincter surface electromyography (sEMG) biofeedback using intravaginal and intraanal probes with embedded electrodes. These devices are particularly well suited to office biofeedback as they produce reliable measures, can easily be inserted and removed by the patient privately with a minimum of discomfort and can be used on a daily basis for home training with a portable take-home sEMG trainer designed for this purpose. Once the appropriate physiological measurement has been selected, in this case external sphincter sEMG, the measured response undergoes electronic processing including analog to digital conversion, amplification, filtering and mathematical reintegration to immediately form a visual, auditory or other sensory signal which is fed back to the patient. In this way biofeedback makes it possible to learn control over physiologic responses of which, ordinarily, we have little or no awareness and consequently are unable to control.
In order for biofeedback to be effective several prerequisites must be met. There must be a measurable response functionally related to the symptoms under treatment, in this case the sEMG response of the external urethral/anal sphincters. This response must have variability and a perceptible sensory cue associated with this variability, in this case the ability to sense changes in the activity level of the sphincter muscle. The ability on the part of the patient to voluntarily change the status of the measured response, that is in this case the ability of the patient to voluntarily contract and relax the external sphincters. These last two requirements also involve an intact nervous system permitting afferent sensory awareness and efferent motor control. Finally, since biofeedback is an active process of learning, in this case sphincter neuromotomotor control, it requires an attentive and motivated patient. The goals of training will vary according to the specific symptoms of the patient. For example, disorders of retention, including urinary retention and constipation, are often associated with chronic or situational excess tone or hypertonicity of the external sphincters. In this case the goal of training is to voluntarily relax the sphincter during elimination. For disorders of incontinence the goal of training is to inhibit bowel or bladder sensory urgency and to voluntarily contract external urethral/anal sphincters to prevent involuntary loss associated with voiding urgency or episodes of elevation of intraabdominal pressure which could lead to involuntary expulsion of urine or feces. Patients acquire these self regulation skills in outpatient training and home training sessions. Through repeated practice and implementation of the learned skills they improve control over the responses that affect continence.
Anatomy and Physiology of Micturation and DefecationMicturation:
The lower urinary tract includes the distal portions of the ureters and their ureterovesical junction at the detrusor muscle. The detrusor is smooth muscle and forms the bladder wall. It provides the propulsive force that expels urine through the urethra. The prostate in the male and the smooth and striated muscle of the urethra are also part of the lower urinary system. The pelvic floor muscles connect to and support the bladder neck, the vaginal and the anal canal. The pelvic floor muscles have significant influence on bladder control. A complex set of learned and reflexive control mechanisms govern the functions of urine storage and micturation. At maturity micturition is coordinated at several levels of the nervous system extending from the frontal lobe and sensorimotor cortex to the peripheral nervous system. Normal bladder function depends upon the integrative functions of the frontal lobes, the sensorimotor cortex, the thalamus, the hypothalamus, the basal ganglia, the cerebellum, specific centers in the brain stem, the spinal cord, nerve roots and the peripheral nerves. The process of urine storage and micturation also involves efferent and afferent activity from the sympathetic and parasympathetic branches of the autonomic nervous system. When experiencing a need to urinate the sympathetic and somatic activity diminish and parasympathetic activity increases. This causes detrusor contractions. The brain stem and cortical centers mediate these peripheral processes. With learning, the cerebral and cerebellar influences become the predominant inhibitory control over bladder contractions.
Hald and Bradley (1982) reviewed the components that interact to maintain normal bladder functioning. These factors help understanding the pathophysiology of various types of voiding dysfunction. To maintain continence during filling the pressure within the bladder must remain low compared to urethral pressure. Disruption of the low intravesical pressure by bladder noncompliance can lead to urine overflow. The base of the bladder is the bladder neck or trigone which is flat during urine storage and forms a funnel during voiding. The integrity of the vesicourethral angle during storage provides a barrier to urine loss when pressure is transmitted to the bladder and the bladder neck through activities which increase intra abdominal pressure, such as laughing, coughing, sneeing, standing and lifting. The proper functioning of the bladder neck depends on urethral pliancy, anatomic placement (Tanagho, 1991) of the bladder neck and sufficient support of the levator ani muscle. Smooth muscle comprises the innermost layers of the bladder neck and the proximal urethra. Appropriate functioning of the urethra depends on the integrity of these smooth muscles. Striated muscles in the distal one third of the urethra, periurethral area and levator ani complex are critical for bladder support and urethral closure. Collagen and elastin connective tissue within the urethral tissue are critical in maintaining the compliance of the urethral lumen for purposes of closure. Vascular, epithelial and hormonal factors also play an important role in the compliance of the urethra. Finally, all of these peripheral factors are interdependant with both central and peripheral nervous system factors that control their functions.
Defecation:
The rectum is the most distal portion of the colon. It extends from the sigmoid colon to the anal canal, is approximately 12-15 centimeters in length and consists of circular and longitudinal smooth muscle layers. When gas or feces move into the rectum it expands to accommodate the larger mass and sensory nerve cells within the rectal walls provide a subjective sensation of fullness and the urge to defecate. Contraction of the colon and rectum provide the propulsive force to move stool toward and through the anal canal. The anal canal pierces the levator ani posteriorally. The levator ani, or pelvic floor muscles form the floor of the bony pelvic and surrounds the urethra, vagina and anal canal providing support for all these lower abdominal organs and interdigitating with fibers of the external urethral and external anal sphincters. The puborectalis portion of the levator ani forms a sling that extends from the pubis around the junction between the rectum and the anal canal and back to the pubis. This muscle is normally in a state of contraction during the storage phase and maintains an acute angle between the rectum and anal canal and is a critical factor in stool continence (Henry & Swash, 1985). The terminal portion of the rectum narrows into the anal canal. The anal canal is approximately 3 cm in length. It consists of the internal anal sphincter, a thickening of the smooth muscle extending down from the distal portion of the rectum, which is maximally contracted during storage, and the external anal sphincter, consisting of bundles of striated muscle fiber, including the deep , superficial and subcutaneous bundles. The external anal sphincter and levator ani maintain minimal activity during the storage phase. The internal anal sphincter relaxes several times an hour allowing small amounts of stool into the anal canal. The walls of the anal canal are densely packed with receptors allowing for the discrimination of gas, liquid and solid. When the internal anal sphincter relaxes for the passage of stool into the anal canal the external anal sphincter must contract to prevent fecal incontinence. This contraction is so highly learned it is often believed to be an unconditioned reflex. However it has been demonstrated that this response does not occur if the individual does not sense rectal distension even when the distension produces internal anal sphincter relaxation, showing this to be a learned response (Whitehead, Orr, Engel and Schuster, 1981). When defecation is desired the puborectalis, internal anal sphincter and external anal sphincter relax in a coordinated fashion and completely for the unrestricted passage of stool.
Pelvic Floor Muscle Anatomy:
Anatomy of the human pelvic floor musculature has been extensively has been extensively studied and described (Pernkopf, 1964, Critchley, Dixon and Gosling, 1980).
The bilateral muscles of the levator ani meet in the midline to form the pelvic diaphragm across the floor of the pelvis. The urogenital hiatus and the anal hiatus pierce through the pelvic diaphragm. The levator ani muscle includes the more anterior and inferior pubococcygeus and the more posterior and superior iliococcygeus. The pubococcygeus attaches to the dorsal surface of the pubic bone and laterally to the arcus tendonius levator ani, or muscle white line. It forms a sling around the anus, prostate or vagina, and urethra. The most anterior fibers are the pubovaginalis, and the more posterior fibers are the puborectalis. The posterior of the levator ani, the iliococcygeus muscle, anchors above the tendinous arch of the levator ani muscle and to the spine of the ischium and below attaches to the anococcygeal body and to the last two segments of the coccyx. The coccygeus muscle, also referred to as the ischiococcygeus, lies adjacent to, and forms a continuous plane with, the iliococcygeus. Laterally the coccygeus is anchored to the spine of the ischium and the fibers of the sacrospinous ligament. Medially it fans out to end on the margin of the coccyx and on thie side of the lowest piece of the sacrum. The Internal obturator muscle forms the muscular sidewalls of the pelvis and narrows down to a tendinous band which exits the pelvic below the ischial spines to attach to the greater trochanter of the femurs. The bulbospongiosis, ischiocavernosus and transversus perinei superficialis muscles on each side of the body form a traingle. The medial leg of the triangle is the bulbospongiosus, also known as the bulbocavernosus or the sphincter vagina which surrounds the vaginal opening. This muscle attaches anteriorly to the copora cavernosa clitoridis with a muscular fasciculus that also crosses over the body of the clitoris and compresses its deep dorsal vein. Posteriorly the bulbospongiosus anchors to the perineal body where it interdigitates with the external anal sphincter and the transversus perinei superficialis. The ischiocavernosus, also known as the erector clitoridis, is located along the lateral boundary of the perineum next to the bony ridge of the anterior pubic ramus between the pubic symphysis and the ischial tuberosity. The ischiocavernosus anteriorally blends with the sides of the crus clitoridis and posteriorly it anchors to the surface of the crus clitoridids and to the ischal tuberosity. The trasversus perinei superficialis muscle spans the perineum laterally between the ischail tuberosities joining the sphincter ani and the bulbospongiosi in the midline at the perineal body.
Click images to enlarge

Inferior to superior view of the pelvic floor musculature as seen from below in the supine female
Types of Urinary Incontinence
Urge incontinence is the involuntary loss of urine associated with an abrupt and strong desire to void. Urge incontinence is usually associated with the urodynamic findings of involuntary detrusor contractions. Urgency in the absence of detrusor contractions is often referred to as sensory urge contrasted with detrusor hyperactivity or motor urge. Ideopathic detrusor hyperactivity is referred to as detrusor instability while detrusor contractions caused by neurologic deficits are referred to as detrusor hyperreflexia or neurogenic bladder (Abrams, Blaivas, Stantan and Andersen, 1988). In patients with neurogenic bladder urge is often accompanied by external urethral sphincter dyssenergia or an inappropriate contraction of the external sphincter which can cause some degree of urinary retention. Urge may also result from involuntary urethral relaxation, known as urethral instability. Urgency can also occur with detrusor hyperactivity with impaired bladder contractility (Resnick and Yalla, 1987) which leads to urge incontinence and elevated post void residuals (PVR’s).
Stress incontinence is the involuntary loss of urine associated with coughing, sneezing, laughing or other physical activities which increase abdominal pressure. This symptom may be confirmed by observing urine loss coincident with an increase in abdominal pressure, in the absence of a detrusor contrction or an overdistended bladder. Most commonly the abdominal pressure causes hypermobility or significant displacement of the urethra and bladder neck during exertion. Stress incontinence can also result from urethral sphincter deficienty (ISD) due to congenital weakness or acquired, most often associated with multiple anti-incontinence surgeries (Blaivas, 1985).
Overflow Incontinence is the involuntary loss of urine associated with overdistension of the bladder. This may present as constant dribbling or may have urge and stress incontinence symptoms. Overflow incontinence may result from an acontractile bladder due to drugs, spinal cord injury, neuropathy, or fecal impaction or may be due to bladder outlet or urethral obstruction leading to an overdistended bladder and overflow.
It is not unusual for patients to present with a combination of both urge and stress incontinence. When symptoms of both types of incontinence are present it is referred to as mixed incontinence.
Finally it should be noted that male urinary incontinence is most often associated with prostate surgery, primarily a radical prostatectomy for prostate cancer.
Types of Fecal Incontinence
There are five major types of deficits associated with fecal incontinence. First, with loss of rectal sensation due to sensory nerve damage or repeated rectal hyperdistension from chronic constipation, the external anal sphincter does not contract with internal anal sphincter relaxation leading to fecal incontinence. Second, a weak external anal sphincter, often resulting from prolonged second stage labor, surgery, radiation or rectal tearing during childbirth can lead to fecal incontinence because the descending stool pressure cannot be offset by the pressure of the external anal sphincter. Third, weak internal anal sphincter tone often resulting from rectal surgery or chronic dilation from constipation. Individual with a weak internal anal sphincter but adequate external anal sphincter may experience leakage of liquid stool or gas which in small amounts will not produce a distension stimulus and therefore will not lead to an offsetting external anal sphincter contraction. Fourth, reduced capacity of the rectum results from rectal noncompliance. This will result in frequent, loose bowel movements often associated with strong bowel urge and involuntary loss or explosive bowel movements. Fifth is a pattern of uncoordinated or paradoxical striate muscle contractions (i.e. external anal sphincter or puborectalis) often referred to as sphincter dyssenergia in the external anal sphincter or paradoxical or nonrelaxing puborectalis in the puborectalis muscle. Failure of appropriate relaxation in these muscles leads to obstructive constipation. Although this is a disorder of retention, not incontinence, this condition can lead to pelvic floor nerve damage through straining which can cause incontinence. Also chronic constipation can lead to rectoceles, prolapse, and hemorrhoids often corrected by surgeries which lead to fecal incontinence. Finally patients can present with both sphincter weakness problems and disordered defecation patterns leading to both fecal incontinence and incomplete evacuation with postdefecation seepage.
Intake ProceduresHistory
Patient evaluation should start with a history of the patient’s urinary and bowel patterns and symptoms. Any pertinent medical history should be reviewed including hospitalizations, surgeries, family history, chronic illness, medication and allergies. Urinary and/or bowel symptoms should be evaluated in the following areas:
Symptom onset, duration and course of symptoms.
Precipitating events
Frequency and volume of voluntary and involuntary voids (voiding diary)
Patterns of straining during voids, pain, and incomplete voids with incontinence after voiding
Food and fluid intake as a factor in voiding
Occurrence of urgency and control of voids or flatus
Timing of incontinence, e.g. nocturnal, diurnal, food/fluid ingestion, activities, emotional state, etc
Type and number of protective pads used
Medication being used such as bladder stabilizing medications or laxatives
Pelvic floor, penile/vaginal or rectal pain
Suprapubic or abdominal cramping
Physical Examination
Clinical practice recommendations for identifying and evaluating urinary incontinence have been made by the United States Department of Health and Human Services (Agency for Health Care Policy and Research Pub. No. 92-0038, 1992). The physical examination should include andominal exam to detect masses, suprapubic tenderness or fullness and estimation of urinary flow and post void residuals or fecal matter retained. Genital examination in men to detect abnormalities of foreskin, glans penis and perineal skin. Pelvic exam in women to assess perineal skin, atrophy, prolapse, pelvic mass, paravaginal muscle tone, urethral hypermobility and bladder beck angle. A rectal exam should be conducted for perineal sensation, sphincter tone, bulbocavernosis reflex, fecal impaction, rectal mass and prostate status in men. If indicated a general exam should be conducted to detect edemetous conditions and neurological abnormalities.
Additional Tests
Additional Urinary testing may include post void residual (PVR) estimation, provocative stress testing, urinalysis, urine cytology, testing for blood urea nitrogen (BUN), voiding record, evaluation of environmental and social factors and observation of urination to detect hesitancy and straining. Specialized testing may include uroflow, cystometry, urodynamics, urethral pressure profilometry (UPP), endoscopy and upper and lower urinary tract imaging. In addition, pelvic floor muscle sEMG has been demonstrated to be clinically reliable and predictively valid in identifying subtypes of urinary incontinence (Glazer, Romanzi, and Polaneczky, 1999, Romanzi, Poleneczky, and Glazer, 1999) and therefore should be a standard part of an initial evaluation for voiding dysfunctions.
Additional testing for fecal incontinence or retention is most often conducted using a Schuster probe, which is a type of anal manometry or pressure reading. This type of assessment provides information on rectal sensation, integrity of reflexes such as internal anal sphincter inhibitory reflex and rectosphincteric reflex response to rectal distension as well as external anal sphincter squeeze pressure and maladaptive abdominal pressure associate with voluntary contraction of the external sphincter. Imaging of the lower gastrointestinal tract can also be conducted using defecography.
In the office practice of biofeedback it is important that patients be fully medically evaluated prior to initiating treatment so that by history and examination all physiological, anatomical, pharmacological, neurological, etc factors can be identified and appropriately treated or ruled out. It has been claimed that external urethral and anal sphincter surface electromyography and biofeedback is a noninvasive, benign intervention, and therefore cannot be contraindicated and does not require prior medical consultation. However, this author strongly believes in requiring patients to undergo appropriate medical evaluation prior to initiating biofeedback as failure to do so may lead to delay in the identification and appropriate treatment of medical conditions including degenerative neurological diseases such as diabetic neruopathy or multiple sclerosis, a wide range of infectious diseases, or even the identification of potential malignancies, all of which may manifest as voiding disorders.
Surface Electromyography Apparatus and Signal Analysis
The sEMG signal is the electrical activity associated with the neuromuscular activation of a voluntary muscle. This signal is highly complex and multiply determined by phenomenon such as anatomical and physiological properties of the striate muscle, central and peripheral nervous system control over the muscle, and the instrumentation and signal processing used to measure the activity. It is imperative that the practitioner of surface electromyographic biofeedback have an understanding of sEMG instrumentation and signal processing (Basmajian, and DeLuca, 1985). In selecting electrodes, instrumentation and signal analysis characteristics for pelvic floor muscle sEMG the user must be familiar with all of the following:
Instrumentation characteristics:
Characteristics of surface electrodes including material, shape, size, spacing of sensor plates, orientation relative to muscle striations, signal stability, response to environmental noise, response to tissue impedence factors and sensor maintenance factors for repeated usage.
Analog to digital conversion rates when using computer based equipment.
Factors relating to electronic pre-amplifiers and amplifiers including noise characteristics, signal to noise ratio, gain, common mode-rejection ratio, input impedance and bias, and bandwidth
Signal Processing characteristics:
Signal rectification preferably by full-wave rectification.
Signal integration of rectified digitized signal preferably by the Root Mean Square (RMS) method.
Frequency domain analysis of the sEMG signal by zero crossing or Fast Fourrier Power Density Spectral Frequency Analysis.
To summarize the important characteristics of instrumentation and signal processing the reader should look for intravaginal or intra-anal sensors constructed with longitudinal sensor plates embedded in a probe which assures the locational stability of the sensor plates relative to the pelvic floor musculature. This will reduce the low bandpass filtering effects associated with the physical relationship between the active bipolar sensors and the active striate muscle. The electrode-electrolyte interface should be consistent over measurements to reduce the high pass filtering effects of this interface. Therefore patients should be told not to introduce any chemical into the vagina or anus for 24 hours prior to the sEMG evaluation. With regard to signal processing, rectification should be full wave. Analog to digital conversion should be at 2000 samples per second per channel. Reintegration should utilize the true Root Mean Square (RMS). Sensor gain should be X500 and input impedance should be greater than one million MegaOhms. Common Mode Rejection Ration (CMRR) should be greater than or equal to –140db from 20 to 500Hz with a 50Hz or 60Hz line filter (depending on the power source of the country in which the instrument is being used). Input bias current should be less than 1 picoAmp and noise less than 0.1uv. Bandwidth should utilize wide bandpass settings of 20Hz +/-5Hz to 500Hz +/-50Hz. These signal processing characteristics will assure accurate, reliable, high quality sEMG readings which will permit meaningful comparisons both within patients over sessions and between patients. The instrumentation selected should have two sEMG channels so the user can measure both pelvic floor muscle and selected accessory muscles which may be helpful in teaching the patient the correct activation of the pelvic floor muscles. In the selection of equipment the user must also keep in mind the necessity of using assessment protocols for meaningful data comparisons and thus the instrumentation used must either permit the user to program protocols or must have application software protocols available for pelvic floor muscle assessment.
Click to enlarge images

Myotrac3 for in-office sEMG pelvic floor muscle evaluation shown above with vaginal sensor and serial port connector with fiber optic cable.
Software Protocol
A protocol is a fixed sequence of events and operationally defined measurements of those events. An sEMG protocol defines a fixed sequence of muscle contraction and relaxation and includes statistical measurements derived from segments of the sEMG signal. The following sequence of activity and measurements are recommended for assessment of the pelvic floor muscle:
Sixty second preliminary resting baseline with measurements of average integrated sEMG rms amplitude, standard deviation and coefficient of variance (i.e. signal standard deviation divided by signal amplitude). This permits an initial assessment of the resting amplitude and variability of the pelvic floor muscle.
A series of 5 rapid contractions or "flicks", each contraction preceded by a ten second rest period. Measurements include rest period average amplitude and standard deviations and flick average peaks and average onset time. This protocol segment assesses both the maximum amplitude and speed of onset of the phasic or fast acting muscle fibers and their effect on resting potential.
A series of 5 ten second contractions, each contraction preceded by a ten second rest period. Measurements include for rest and work periods the average signal amplitude, standard deviation and coefficient of variance. For contractions, measurements also include either median power density spectral frequencies or zero crossings as well as average contraction onset and release times. This protocol segment is referred to as the tonic muscle fiber activity. It helps define the type of muscle fibers participating in a contraction and to what degree, as well as the effects of tonic contractions on the resting potential.
A single 60 second sustained contraction preceded and followed by a 10 second rest period. Measurements include for both rest periods and the work period the average signal amplitude, standard deviation and coefficient of variance. For contractions, measurements also include median power density spectral frequencies or zero crossings as well as signal amplitude decline during the contraction. This protocol segment is referred to as the endurance muscle fiber test. It helps to assess the type of fibers participating in a sustained endurance contraction, the effects of this contraction on the resting potential, and the degree of muscle fatigue which occurs during the endurance contraction.
Sixty second post baseline resting period with measurements of signal average amplitude, standard deviation and coefficient of variance. This segment defines the resting amplitude and variability of the muscle after the series of protocolized contractions have been conducted.
This protocol was first described in 1997 (Glazer and Marinoff) and is known as the Glazer Protocol. This protocol not only provides a fixed sequence of muscle actions and their measurements but also provides a database of sEMG readings for normal subjects and those with a range of pelvic floor muscle related dysfunctions.
Conducting the Surface Electromyographic Pelvic Floor Muscle Assessment:
Prior to the conduct of the pelvic floor muscle surface electromyographic assessment it is important to first educate the patient on the procedure. A description of the gross anatomy of the pelvic floor muscle structures and the lower abdominal viscera is provided to the patient. The functions of the pelvic floor muscles; sexual, sphincteric, and support, and their relationship to fiber types are then discussed. Basic mechanisms of urine/stool storage and elimination are reviewed. It is critical for the patient to understand the structures and functions of the pelvic floor musculature and how they relate to their clinical symptoms in order to achieve maximum therapeutic benefit. Next the patient is provided information on surface electromyography and given information on the types of measurements that will be conducted. It very helpful to provide patients with normative readings for each of the measures which will be conducted so that they are able to see, along with the clinician, any deviations from normal readings which appear during the conduct of the evaluation. Normative readings will vary depending on the type of hardware, signal processing and software which is used. In general both pre baseline and post baseline average resting tone is 2uv, with a standard deviation of .2uv, peak flicks average 30-35uv with onset times of .2sec. Tonic contractions average 20-25uv with standard deviations of 2.5uv, median spectral frequencies of 120Hz, and onset and recovery times of under one second. A general rule is that the resting amplitude during the tonic contraction phase should average no more that 10% of the average tonic contraction amplitude. Endurance contraction amplitude should be 20uv with median spectral frequencies of 125Hz and should show little to no amplitude decline throughout the 60 second endurance contraction. At this point the patient is shown the vaginal or anal sensor and given detailed instructions on the insertion of the sensor including the orientation of the sensor and the appropriate use of lubrication.

sEMG anal sensor (top) and vaginal sensor (bottom) with grounding patches.
The patient then privately self -inserts the sensor as instructed and returns to the procedure room fully clothed with the sensor wire available to be connected to the electromyograph. Both the clinician and the patient must be able to simultaneously view the sEMG tracing on the screen. This can most easily be accomplished by using two screens, one for the patient and one for the clinician.
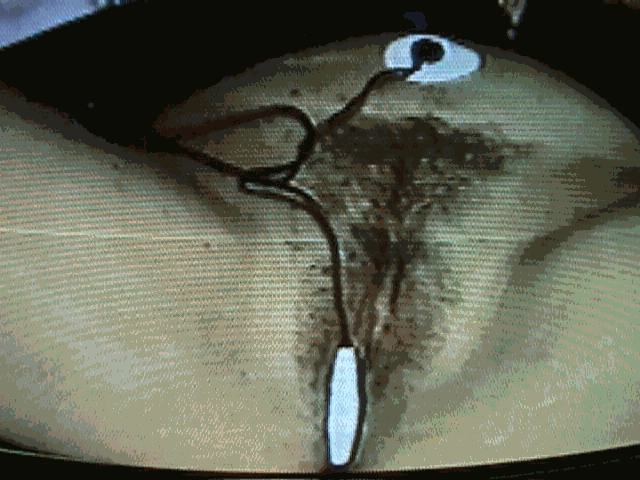
Patient with correct placement of intravaginal sEMG sensor with ground pad attached.
Positioning of the patient for evaluation and instructing the patient on the correct manner to contract and relax the pelvic floor muscles is important as this can substantially alter the sEMG readings. Initial readings are best made with the patient in a reclining position with the top half of the body at approximately a 135 degree angle to the bottom half. The legs should be rotated outward with the heels slightly separated. Degree of rotation will vary amongst patients and should be set to the patients’ comfort. While determining the best position for the patient the sEMG should be monitored as it may significantly vary across different positions. For example, the greater the degree of hip rotation the higher the pelvic floor muscle sEMG as hip rotation induces activation of the internal obturators which in turn connect to the anterior portion of the pubococcygeus where they share a fascial connection at the archus tendonius levator ani or muscle white line. While adding activity in this manner to the pubococcygeus muscle may enhance the patients’ sensory awareness of pelvic floor contractions it can also produce a falsely elevated resting tone leading to an incorrect diagnosis of resting hypertonicity. Patients may also be tested and may conduct a portion or all of their home exercises in varying positions including reclining, sitting and standing. This will be determined by the nature of the patient’s symptoms. Type II stress incontinence, in which involuntary urine loss is associated with acute episodes of intra-abdominal pressure such as that produced by laugh, cough, sneeze or lift, occurs almost exclusively in the standing position. Therefore, at least part of the assessment and rehabilitation of the pelvic floor muscle in these patients must be conducted in the standing position. Urge incontinence, involuntary urine loss associated with a strong urge to void, can occur in any position and therefore these patients must be assessed and trained in all positions.

Patient positioned correctly with sEMG intravaginal sensor inserted. Note the
position of the legs slightly rotated externally and the elevation of the back.
Also note the presence of two screens, one for the patient to view the sEMG and the
laptop computer from which the clinician controls the protocol administration.
Once the patient has been correctly positioned the process of teaching the patient the correct use of the levator muscles begins. While the activation of most striate muscles can be accomplished by instructing the patient to perform a certain movement, this is not true for the pelvic floor musculature. Furthermore, the instruction to "contract your pelvic floor muscles" is of little value as most patients will have no idea how to do this. It is best to start with an instruction such as "hold in as if you are experiencing and urge to urinate and have a bowel movement at the same time". This instruction is most likely to lead to the simultaneous contraction of both the anterior and posterior aspect of the pubococcygeus muscle, thus providing the most accurate sEMG measurement of the contractile capacity of the muscle. Training the correct use of the pelvic floor muscles is a highly variable phenomenon, much like teaching a child to ride a bike. Some get on and ride off in minutes while others struggle and never truly attain the skill. Acquiring pelvic floor muscle control is neither an intellectual/cognitive task nor one which simply requires maximum undifferentiated muscle effort. It is a refined neuromuscular skill, which, like bicycle riding, or playing tennis can be acquired only by diligent and reliable practice. Much like controlling the bicycle or the tennis ball for the first time, patients are usually aware of correctly activating the pelvic floor musculature. The usual description spontaneously offered by patients is a sensation "lifting" or "sucking up" of the sensor. This sensation may be very subtle at first and hard for the patient to produce on command. With training patients become reliably able to contract the pelvic floor muscle correctly. The clinician can use the sEMG pelvic floor muscle signal to aid the patient in learning the correct activatin pattern of the muscle. The correct use of the muscle will result in an elevation and stabilization of sEMG which can be quickly recognized by both clinician and patient. It is the essential change which the muscle undergoes during this learning process which permits the patient to use the muscle in a manner which can control dysfunctional urogenital symptoms. In the initial evaluation we simply start the learning process.
In most patients first asked to activate their pelvic floor muscles you will likely see significant co-contractions occurring in other muscle groups. This phenomenon may be restricted to the pelvic region, including gluteal maximus, transverse abdominals and leg adductors, or it may involve more pervasive whole body tension, including breath holding. Creating the correct balance of muscle usage for each patient is critical for the initial evaluation and to get the patient started correctly in the conduct of their home exercises. Not surprisingly, it is patients who have weak pelvic floor muscles and/or poor ability to sense pelvic floor muscle contractions who are most likely to engage in this excessive contractile pattern. In teaching patients the correct muscle usage pattern, a balance must be struck. Excluding all co-contractions will lead to submaximal contractions of the pelvic floor and will therefore limit the muscle rehabilitation, which is best achieved with maximal contraction force. On the other hand, excessive undifferentiated recruitment of large muscles will overwhelm any sensory awareness of pelvic floor muscle contractions and prevent the patient from learning appropriate control patterns over the target muscle group. In a balanced approach the patient is taught to focus on a maximum force pelvic floor muscle contraction while permitting natural overflow or co-contractions in a limited manner (Glazer and MacConkey, 1996). In this fashion patients can best engage in rehabilitation of pelvic floor musculature while limiting co-contractions. In order to facilitate this learning the clinician can use sEMG measurements of co-contracting muscles or can simply manually palpate to help the patient limit any excessive overflow.
The patient is provided only enough instruction and education to permit the conduct of a valid pelvic floor muscle assessment protocol. This process should take no more than five minutes. At this point, although the patient remains far from reliable in their use of the pelvic floor musculature, the clinician should proceed with the protocol. The patient should be told in advance exactly what series of muscle activity they will be asked to perform. Patients should also be instructed to limit any movement as this may lead to artifacts in the sEMG recordings. In order for a protocol to produce data for reliable within patient over sessions, and between patient comparisons, the protocol must be run in exactly the same manner every time. Excessive pauses between segments to conduct discussions with the patient should not occur. Patients should be told to hold any questions until the completion of the protocol. The entire Glazer protocol, described earlier, takes approximately five minutes to conduct. Upon completion of the protocol the clinician should immediately complete any information, including clinical notes, required by the software, in order to print out a complete report. The instrumentation and software which you choose should have the capacity to print out a complete report, including sEMG tracings, statistical summaries and clinical notes. An example of such a printout follows.
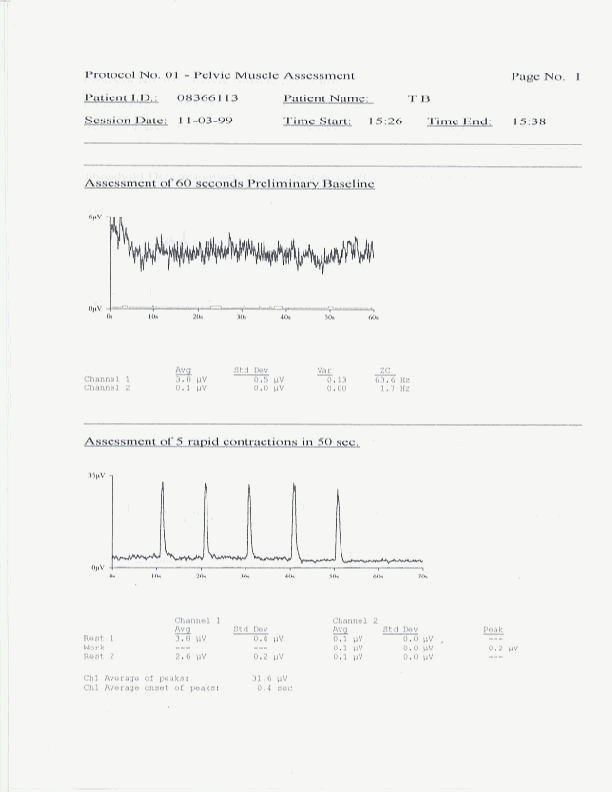

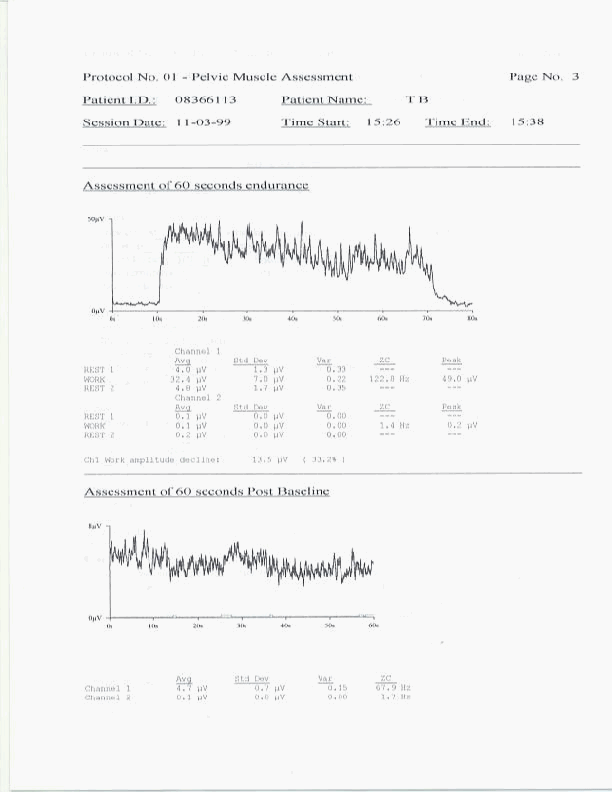

A complete copy of such a printout should be provided to the patient on each office evaluation. At this time the clinician should review all the findings with the patient and compare the findings to those of previous sessions when appropriate. Findings reviewed should include interpretations of "flick" peaks and onset time, "tonic" rests and resting variability, contractile onset and recovery times, contractile amplitude, variability and spectral frequency, and "endurance" contraction amplitude, variability, spectral frequency and fatigue. In this manner all the sEMG muscle abnormalities can be identified for the patient and changes in these measures can be followed over time. These readings are used by the clinican to modify any patient home exercise instructions so that the patient can clearly understand the purpose of all exercise modifications.
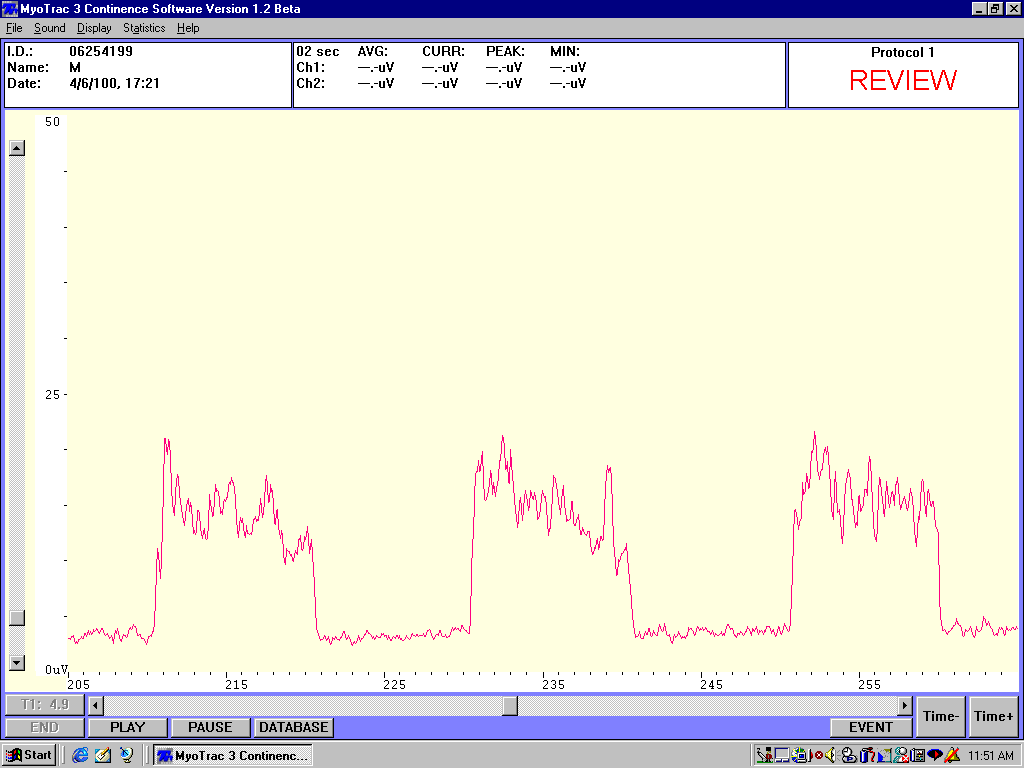
Sample sEMG tracing showing resting hypertonicity and instability, low amplitude contractions with slow onset and recovery with poor control and low spectral frequencies
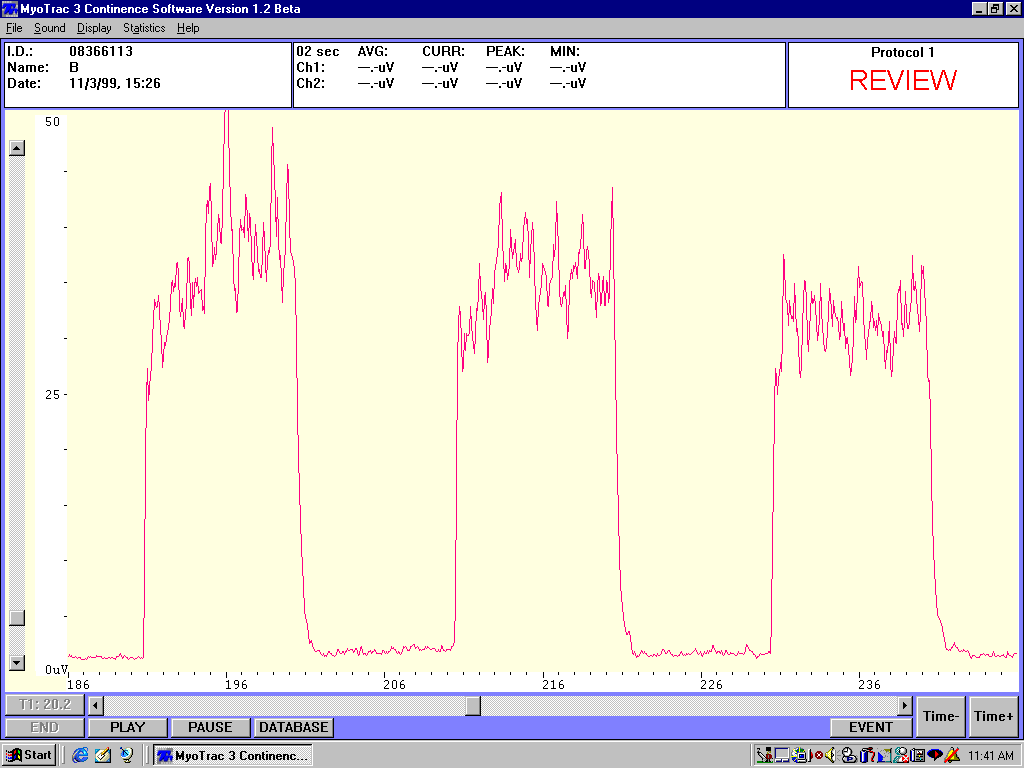
Sample sEMG tracing showing stable low amplitude resting potential with high amplitude contractions and rapid onset and recovery with good control, no fatigue and high spectral frequencies.
Following the initial evaluation, assuming sEMG muscle abnormalities consistent with patient symptom reports are found, the clinician will then demonstrate the use of the sEMG home training device and prescribe the home training regimen. All patients are initially prescribed home training of 10 second maximum pelvic floor muscle contraction followed by 10 seconds of relaxation for a total of 60 repetitions, that is twenty minutes, twice a day, preferably morning and evening. All exercises are initially prescribed to be performed in the reclining position. Some elderly, frail, or weak patients may be initially unable to complete the full prescribed number of repetitions and may need to build up to the full twenty minute exercise periods. It is also important for the clinician to assess the likelihood of compliance with home exercise. Approximately 25% of patients undergoing initial evaluation and prescription of home training will drop out of treatment. Thus, patients should be thoroughly assessed for their motivation and ability to reliably comply with the home training protocol to minimize therapeutic failures due to noncompliance. It is best to see patients once a week for the conduct of an office evaluation. This helps to support patient compliance with home exercises and allows the clinician to follow the patient’s sEMG muscle changes and symptomatic progress.

U-Control sEMG biofeedback home trainer pictured here with vaginal sensor and grounding pad.
Office Follow-up visits:
Modification of prescribed home training is done on an individualized basis. It is rarely advisable to change the basic prescription of 10sec work/10sec rest with 60 repetitions, twice a day. However, many other exercise parameters and factors may be modified based on office assessment findings over the course of treatment. For example, intentional use of acessory muscles may be used for persistent weakness. Having the patinet focus on rapid initiation of voluntary contractions can be used to address patients reports of leakage even while initiating a pelvic floor muscle contraction. The patient may instructed to focus on reducing the variability of the contraction if small amounts of loss occurring with sustained contractions. Home training position may be altered, as the patient gains contractile amplitude and control more of their home training can be conducted in the standing position.
Pelvic floor muscle rehabilitation is only the first step. As the patient increased their contractile speed, amplitude, and control they will need instruction in the application of the external sphincter to control involuntary loss. Patients with stress incontinence must be told that they need to initiate a voluntary contraction of the pelvic floor muscles just prior to the occurrence of acute intra-abdominal events such as a laugh, cough, or sneeze. Urge incontinence patients must be instructed to remain still when they experience a strong urge and to contract and hold the pelvic floor muscles until the urge goes away. This can be quite difficult for patients as it is counterintuitive to the natural desire to quickly get to the toilet in response to an urge. This will increase the likelihood of involuntary loss because patients cannot effectively contract their pelvic floor muscles while walking or running to the toilet. Using pelvic floor muscle contractions to inhibit the detrusor activity associated with voiding urgency can be used to progressively increase the time between voids in those who suffer urinary urgency and frequency. In such patients voiding is conducted on a timed basis, slowly increasing the inter-void intervals. Also voiding patterns, diet, fluid consumption, straining, and dysfunctional muscle use patterns must be addressed at each follow up session. Symptom changes must be assessed with voiding diaries and pad test.
Disorders of Retention
In disorders of functional urinary retention or constipation a complete sEMG evaluation can assist in identifying any sphincteric component of the problem. Most commonly chronic essential hypertonicity, episodic hypertonicity of external sphincters, or learned dysfunctional voiding habits, with or without an identifiable precipitant, can lead to disorders of retention. Excessive pelvic floor muscle activity associated with voiding dysfunction is referred to as sphincter dyssenergia. The sEMG training for pelvic floor muscle dyssenergia is similar to training for incontinence but during the conduct of prescribed muscle exercises greater emphasis is put on improving the patient’s discrimination of low levels of pelvic floor muscle activity. As with other forms of progressive muscle relaxation the clinician seeks to lower the pelvic floor muscle activity by having the patient contract to maximum amplitudes followed by a period of relaxation. The resting tone of the muscle will likely be reduced after a series of maximum contractions allowing the patient enhanced awareness of levels of relaxation. The same home prescription of 20 minutes twice a day of 10 second contract/10second relax is used. The patient is focused on quickly and completely releasing the maximum contraction and becoming aware of small differences in post contraction pelvic floor muscle relaxation. As with incontinence treatment these exercise can advance from reclining to sitting and to standing position. Finally the patient must learn to maintain the greatest levels of relaxation while bearing down with a gentle Valsalva maneuver. This helps the patients ability to generalize the pelvic floor muscle relaxation response to the actual voiding situation.
Application of Pelvic Floor Muscle Surface Electromyography to Urogenital Pain
Within the past decade Glazer and his colleagues have demonstrated the application of pelvic floor muscle surface electromyography in the diagnosis and treatment of a broad range of essential urogynecological pain disorders. This includes conditions such as urethral syndrome, vulvodynia, vulvar vestibulitis syndrome, chronic abacterial prostatitis, or prostatodynia, in men, and levator ani syndrome. The etiology of these disorders is most often associated with tissue irritation or trauma related to infections and/or substances used locally to treat these infections, hormonol changes, dermatologic irritants, surgery, or accidents, but fail to self resolve once the provocation has been resolved. The majority of these patients show significant pelvic floor muscle hypertonicity and resting instability often accompanied by slow recruitment and recover times, contractile weakness and instability with low spectral frequencies. These sEMG abnormalities have been shown to diagnostically differentiate essential vulvar pain patients from both vulvar pain of acute medical origin (White, Jantos and Glazer, 1997) and from normal asymptomatic females (Glazer, Jantos, Hartmann and Swencionis, 1998). The application of sEMG pelvic floor muscle rehabilitation has also been demonstrated to be effective in the treatment of essential vulvovaginal pain disorders (Glazer, Rodke, Swencionis, Hertz and Young, 1995). Long term follow up conducted on patients successfully treated with this protocol has shown the maintenance of therapeutic benefit for three to five years (Glazer, in press). For further information on the application of sEMG to the treatment of pelvic pain disorders visit Dr. Glazer’s website located at http://www.vulvodynia.com
Sample Voiding Record
Name: ___________________________________________________________
Date: ____________________________________________________________
INSTRUCTIONS: Place a check in the appropriate column next to the time you urinated
In the toilet or an incontinence episode occurred. Note the reason for the incontinence and describe your liquid intake (for example, coffee, water) and estimate the amount (for example, one cup).
Number of pads used today _______________ number of episodes _________________
Comments ______________________________________________________________
_______________________________________________________________________
Glossary of Terms
Adduction - To draw towards the midian plane.
Afferent - Sensory signals from the somatic and autonomic nerves that carry information to the spinal cord.
Anal sphincters - The anal canal is surrounded by the voluntary external anal sphincter and the involuntary internal anal sphincter. The internal sphincter is continuous with the smooth muscle lining the colon and the external sphincter is continuous with the puborectalis portion of the levator ani. Discoordination of the two sphincters leads to voiding dysfunctions including constipation and incontinence.
Anorectal monometry - A procedure involving the use of balloons to measure how much pressure can be generated within the anal canal and rectum.
Bladder-sphincter dyssenergia - A discoordinated voiding pattern in which the bladder and sphincter contractions happen simultaneously rather than the sphincter relaxation for urination. This can occur as a learned voiding dysfunction as well as for neurological reasons.
Bladder training - A behavioral program to increase intervoid intervals and enhance bladder capacity, usually involving timed voids as a treatment for urge incontinence.
Bulbocavernosis reflex - This is a relflex contraction of the anal sphincter mediated by the pudendal nerve and produced by squeezing the clitoris or the glans of the penis.
Coccyx - Three to five small fused ridimentary vertebrae forming the lower extremity of the veterbral colum extending from the sacrum
Collagen - Fibrous nonsoluble protein in connective tissue.
Cystometry - A urodynamic evaluation of the bladder volume/pressure relationship assessed by filling the bladder while measuring presure and perineal muscle activity.
Detrusor hyperactivity with impaired bladder control - A neurologic disorder in which uninhibited detrusor contractions occur with bladder contractions that are inefficient in emptying the bladder without the aid of abdominal straining.
Detrusor instability - Involuntary detrusor contractions in the absence of neurological disease.
Detrusor Muscle - Smooth muscle making up the wall of the bladder that contracts to expel the urine.
Elastin - The essential part of the yellow elastic connective tissue made up of protein.
Fast twitch muscle fiber - Anaerobic striated muscle fiber that provides strong fast contractions as compared to aerobic slow sustaining striate muscle fiber.
Hyperreflexia - Involuntary bladder contractions resulting from neurological disease
Levator ani muscle - The large muscle which forms the floor of the pelvis and supports the pelvic viscera. The levator ani is made up of the pubococcygeus, puborectalis and iliococcygeus muscles. The levator ani interdigitates with the external urethral and anal sphincters for continence.
Levator ani syndrome - A number of conditions including coccydynia, proctalgia fugax, prostatodynia, characterised by idiopathic pain in the anal and perianal region.
Micturation - The voiding of urine.
Mixed incontinence - Coexisting stress and urge incontinence.
Nocturia - Excessive urination at night
Nonrelaxing puborectalis muscle - The puborectalis muscle maintains the angle between the rectum and anal canal and prevents stool from passing out of the rectum for continence. This muscle should relax with defecation. Failure of the puborectalis to relax during defecation leads to stool retention or constipation.
Pad test - A test used to measure urine leakage in which the patient records the use of pads which are weighed before and after use.
Prolapse - Prolapse means to move forward, down or out and subsumes several specific conditions including rectocele, cystocele, urethrocele andcystourethrocel involving the movment of the rectum, bladder and urethra. The degree of movement is graded with fourth degree being most severe and involving the extrusion of the organ through the vagina or rectum.
Sensory urge incontinence - Urge incontinence without unhibited bladder contractions.
Trigone - Base of the bladder.
Ureterovesical - Pertaining to the ureters and the bladder.
Urogenital diaphram - A thin sheet of striated muscle stretching between the two sides of the pubic arch.
Urethral syndrome - A diagnosis applied to symptoms of urethral discomfort after ruling out identifiable anatomic defect and infectious conditions.
Valsalva maneuver - A bearing down conducted as a forced exhalation effort against occluded nostrils and closed mouth. This maneuver increases intrathorasic and intra-abdominal pressure.
References:
Visit Dr. Glazer.s website at: http://www.vulvodynia.com
References
Hald, T., & Bradley, W.E. The urinary bladder: Neurology and dynamics. 1987, Baltimore: Williams & Wilkins.
Tanagho, E.A. Retropubic surgical approach for correction of urinary stress incontinence. In D.R. Ostergard and A.E. Bent (Eds.), Urogynecology and urodynamics: Theory and practice (3rd ed.), Baltimore: Williams & Wilkins, 1991
Henry, M.M. and Swash, M., Fecal Incontinence, defecation and colorectal motility. In M.M. Henry & M. Swash (Eds.) Coloproctology and the Pelvic Floor: Pathophysiology and management, pp 42-47, 1985, London: Butterworths.
Whitehead, W.E., Orr, W.C., Engel, B.T., and Schuster, M.M., External anal sphincter response to rectal distension: Learned response or reflex, Psychophysilogy, 19: 57-62, 1981.
Critchley, H.O.D., Dixon, J.S., and Gosling, J.A., Comparative study of the periurethral and perianal parts of the human levator ani muscle. Urol Int 35: 226-232, 1980
Pernkopf, E., Atlas of Topographical and Applied Human Anatomy, Vol. 2. Philadelphia: W.B. Saunders, 1964.
Abrams, P., Blaivas, J.G., Stanton, S.L., and Andersen, J.T. Standardization of terminology of lower urinary tract function. Neurourology Urodynamics, 7: 403-427, 1988.
Resnick, N.M., and Yalla, S.V., Detrusor hyperactivity with impaired contractile function: An unrecognized but common cause of incontinence in elderly patients. Journal of the American Medical Association, 257(22): 3076-3081, 1987.
Blaivas, J.G., Pathophysiology of lower urinary tract dysfunction. Urologic Clinics of North America, 12: 215-224, 1985.
Urinary Incontinence Guideline Panel. Urinary Incontinence in Adults:Clinical Practice Guidelines, Agency for Health Care Policy and Research Pub. No. 92-0038, Rockville, MD, U.S. Department of Health and Human Services, March, 1992.
Basmajian, J.V., and DeLuca, C.J., Muscles Alive: Their functions revealsed by electromyography, Baltimore: William & Wilkins, 1985.
Glazer, H., and Marinoff, S., The Treatment of Vulvovaginal Pain Disorders with Surface Electromyographic Assisted Pelvic Floor Muscle Rehabilitation, Electromyography: Applications in Urology and Gynecology, Biofeedback Foundation of Europe, 1997.
Glazer, H., MacConkey, D., Functional Rehabilitation of Pelvic Floor Muscles: A challenge to tradition. Urological Nursing 16: 68-69, 1996.
Glazer, H., Romanzi, L., and Polaneczky, M., Pelvic Floor Muscle Surface Electromyography: Reliability and Clinical Predictive Validity, J. Reprod. Med. , 44: 9:779-782, 1999.
Romanzi, L., Polaneczky, M., and Glazer, H., Simple Test of Pelvic Muscle Contraction During Pelvic Examination: Correlation to Surface Electromyography, Neurourol and Urodynamics, 18:603-612, 1999.
White, G., Jantos, M. and Glazer, H. Establishing the Diagnosis of Vulvar Vestibulitis, J. Reprod. Med., 42:3:157-160, 1977.
Glazer, H., Jantos, M., Hartmann, E., and Swencionis, C., Electromyographic Comparisons of the Pelvic Floor in Women with Dysesthetic Vulvodynia and Asymptomatic Women, J. Reprod. Med., 43:11:959-962, 1998.
Glazer, H., Rodke, G., Swencionis, C., Hertz, R., and Young. A.W., Treatment of Vulvar Vestibulitis Syndrome with Electromyographic biofeedback of Pelvic Floor Musculature, J. Reprod. Med., 40:4:283-290, 1995
Glazer, H., Long Term Follow Up of Dysesthetic Vulvodynia Patients after Completion of Successful Treatment by Surface Electromyography Assisted Pelvic Floor Muscle Rehabilitation, J. Reprod. Med. , in Press.
Reducing multifetal pregnancy through publicly funded IVF programs
April 26th 2024Learn how a mandatory elective single-embryo transfer policy in publicly funded in vitro fertilization programs significantly decreases multifetal pregnancy rates, offering insights into mitigating risks in assisted reproduction.
Read More
S1E4: Dr. Kristina Adams-Waldorf: Pandemics, pathogens and perseverance
July 16th 2020This episode of Pap Talk by Contemporary OB/GYN features an interview with Dr. Kristina Adams-Waldorf, Professor in the Department of Obstetrics and Gynecology and Adjunct Professor in Global Health at the University of Washington (UW) School of Medicine in Seattle.
Listen
Higher preterm birth risk found following cesarean delivery at full dilation
March 26th 2024Recent research highlights an association between cesarean delivery at full dilation and increased risk of subsequent preterm birth, prompting further investigation into childbirth practices and outcomes.
Read More
